Alithya can help you grow, evolve, and transform your business with Microsoft Dynamics 365
Does your organization struggle with using outdated technology to manage ongoing supply chain disruption, inventory, cost control, workforce management, financial constraints, effective marketing and sales strategy, and more? With Alithya’s strategy and industry specific solutions, coupled with Microsoft’s technologies, you can adapt and innovate across your entire business. Microsoft Dynamics 365 provides a single integrated platform for all your business needs. Alithya has developed many products and industry accelerators to help you get the most out of your investment in Microsoft Dynamics 365.
We provide end-to-end Microsoft implementation and upgrade services to help you define and achieve your financial, operational, and customer engagement goals.
Alithya’s services combined with Microsoft’s technology help you share insights, improve collaboration, enhance security, and access global data centers - generating real business outcomes and value.
Benefit from our award-winning, outcome-driven approach
With Alithya, implementing your Microsoft solutions can mark the start of a transformation journey full of new possibilities.
Robust capabilities across six cloud-enabled Microsoft solutions
Alithya is a top-tier expert across six Microsoft solutions:
Modern work
Boosting productivity and managing hybrid and remote work with Microsoft 365.
Business applications
Automating processes with Microsoft Dynamics 365 and Power Platform.
Data management and AI
Managing and governing data across multiple systems to build analytics and AI solutions.
Security
Safeguarding organizations with integrated security, compliance and identity solutions.
Infrastructure
Accelerating migration of key workloads to Microsoft Azure.
Digital and app innovation
Modernizing existing applications and building cloud native apps.
Alithya CONNECT
Your Microsoft Managed Services Portal
Get the most out of your technology investment with ongoing managed services and support for your Microsoft solutions
Microsoft Dynamics 365 customer relationship management
How do you keep your clients happy? How do you keep them loyal? At Alithya, we understand that oftentimes happy customers equal a successful business. However, businesses often struggle with disorganized customer data, inefficient communication between teams, lack of personalization, and limited visibility in customer interactions. In addition, disjointed workflows and lack of integration with existing tools can hinder efficiency.
Step up your marketing, sales, and service success with automation, data analytics, real-time business intelligence, and end-to-end campaign management. Alithya’s experts can help you make the right CRM choices to create more engaging customer experiences, leverage intelligent sales and marketing, enable proactive customer services, unleash connected field services, and connect with every customer.
Address disjointed customer, sales and marketing data with a unified Microsoft CRM solution.
Close more deals and build lasting connections with a single view of your customers and their future needs. Plan, execute, and analyze campaigns seamlessly across channels for a higher rate of return.
Consistently exceed customer expectations by optimizing resources and assisting technicians efficiently.
Boost service levels across all touchpoints by providing fast access to real-time customer data.



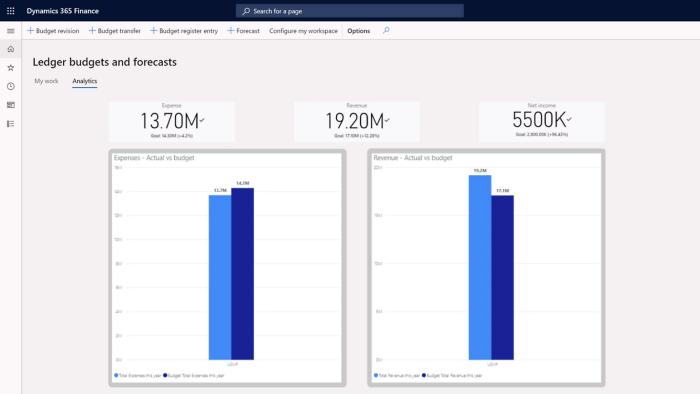
Microsoft Dynamics 365 enterprise resource planning
From finance and supply chain to project and talent management, embed business intelligence, insights, and process automation in the applications your people rely on to accomplish more while delivering strategic outcomes.
Assess financial health and improve controls with embedded analytics and AI-driven insights.
Predict change and respond quickly with transparency, proactive planning, and optimal productivity.
Track progress, assign the right resources, and deliver profitable projects on time and on budget.
Elevate employee recruiting, onboarding, and retention with an integrated, scalable human capital solution.
Leading industry solutions to address daily challenges
Achieve your unique business outcomes with Microsoft technologies and Alithya’s deep industry knowledge.
Elevate the performance of your entire business ‒ from the factory floor to customer service ‒ while addressing compliance, production, and sales challenges. Navigate erratic demand, resource and capacity constraints, strict regulations, and raw material volatility by empowering employees with accurate forecasts, real-time intelligence, best-practice templates, and modern ERP and CRM solutions.
Alithya solutions for all manufacturers:
- Alithya 365 Operations: Consolidate the information you need so you can gain key visibility on quality problems and drive important decisions in your organization to ensure compliance with requirements.
- Alithya 365 Power Apps for Manufacturing: Give your team higher-quality data to plan better, sell more, track assets and warranties, and process returns with our Microsoft Power Apps for manufacturing.
- Alithya 365 DiscreteXpress: Use a pre-configured infrastructure, toolset, and sample data to provide an easy place for discrete goods manufacturers to start their implementation—eliminating costly hours of analysis and training. The result: easily manage production orders, support lean manufacturing processes, forecast sales, configure products and more.
- Alithya 365 EAMXpress: Incorporate leading industry scenarios into a tested and proven asset management template for a quick shop-floor ERP implementation in equipment-focused industries. The result: streamline processes like corrective and preventative maintenance as well as spare parts management.
Alithya solutions by subvertical:
Microsoft Dynamics 365 and our industry expertise can empower you to conquer the challenges of producing and distributing high-quality food. Weather changing consumer preferences, tightening regulations, and rising demand for sustainability with a data-driven culture running on one source of truth.
Take on any challenge, including:
- Regulatory requirements
- Changing consumer demands
- FSA/USDA validation
- Recall and mock recalls
- Quality control
- Mergers and acquisitions
- Traceability
- Supply chain disruptions
Alithya 365 FoodXpress: Integrate more than 1,000 defined processes, data sets and food industry-related settings into Microsoft Dynamics 365 for F&O. The result: better manage batches and lots, co/by-product and catch weight processing, trade promotions, customer rebates and more.
Alithya 365 Dairy: Fulfill dairy-specific regulatory requirements, complex accounting and reporting rules, quality testing demands, traceability needs, and complex formulations/processing requirements.
Manage multiple variables across your product engineering and delivery processes by combining Microsoft Dynamics 365 with industry expertise that chemical manufacturers trust.
Solve challenges, including:
- Growing from mergers and acquisitions
- Changing regulations
- Minimizing raw materials waste
- Managing batch potency
- Accelerating research and development
- Developing sustainable products
Alithya 365 ChemXpress: Streamline chemical-specific procedures and leverage the benefits of your historical data for quick reporting across your organization. Results: easily manage batches and lots, potency, quality, co/by-product processing, customer rebates, trade promotions, compliance tracking, attribute-based pricing and more.
Alithya and Microsoft Dynamics 365 can help you move goods faster with less waste and better compliance. Maximize capacity, comply with regulatory demands, and drive continuous improvement by gaining greater visibility organization-wide with one source of truth.
Tackle these areas:
- Regulatory requirements
- FDA and USDA validation
- Quality controls
- Complete traceability
- Production and R&D management
- 21 CFR Part 11 electronic records
- Sarbanes-Oxley (SOX) compliance
- Good Automatic Manufacturing Practices issue 5 (GAMP 5)
- Security and compliance single sign-on
- Customer-specific requirements
- Expensive R&D process and high production costs
- Counterfeit products
- Supply chain disruptions
- Cyber security concerns
Alithya 365 PharmaXpress: Implement your ERP system properly to meet the needs of unique government regulations for pharmaceutical manufacturers and keep the thorough records needed to say compliant. Results: improved quality management, batch/lot management, compliance tracking, production dispensing and more.
Deliver high-quality products that ensure optimal patient outcomes by extending Microsoft Dynamics 365 with industry expertise that medical device manufacturers can trust. Get the full picture of your business in real time to anticipate and respond to supply chain risks and securely provide innovative life-saving medical devices.
Solve top challenges, including:
- Regulatory requirements and validation
- Complete traceability and quality controls
- Expensive R&D process and high production costs
- Counterfeit products
- Supply chain disruptions
- Cyber security concerns
- 21 CFR Part 11 electronic records
- Sarbanes-Oxley (SOX) compliance
- Good Automatic Manufacturing Practices issue 5 (GAMP 5)
Alithya 365 MedDeviceXpress: Combine lean manufacturing processes with strict regulatory compliance processes to succeed in the complex medical device manufacturing industry. The result: deploy an ERP system on a tighter timeline to meet these requirements and more.
Capture new sources of growth with operational agility and sustainability by blending Microsoft Dynamics 365 with expertise that building products manufacturers trust. Protect your revenue streams and profitability with data-driven smart manufacturing processes and business models supported by one source of truth.
Face your challenges, including:
- Construction market volatility
- Supply chain disruptions
- Tariff changes
- Production planning
- Inventory management
- Quality control
- Sustainability reporting
- Mergers and acquisitions
Keep up with volatile economic growth and surging demand by optimizing Microsoft Dynamics 365 with proven expertise for industrial goods manufacturers. Satisfy customer demand and bring smart manufacturing to life with Microsoft Dynamics 365 and warranties, configuration, Lean, advanced engineering change orders, product lifecycle management, enterprise asset management, field services, and more.
Microsoft Dynamics 365 can help you tackle common challenges like:
- Surging global competition
- Never-ending supply chain disruptions
- Emerging skill gaps
- Reshoring strategies
- Changing consumer demand
- Ongoing cybersecurity
- Needing to embrace new technology
Close more deals, track services, and manage warranties with an integrated Microsoft solutions that covers operations, field service, sales, customer service, project management, and marketing activities. Leverage data more effectively to strengthen relationships with customers and equipment manufacturing partners with relevant opportunities for ongoing growth.
Overcome top challenges, including:
- Labor shortages
- Equipment downtime
- Supply chain disruptions
- Inventory management and services
- Customer relationship management
- Surging demand for new and used equipment
- Lack of adoption of the latest technology
Alithya 365 Power App for Equipment Dealers: Leverage AI and data to analyze equipment sales and service history to target the right prospects and close more business.
Alithya 365 Advanced Field Service: Configure, price, quote and fulfill complex orders based on inventory and build-to-suit configured products, including parts, warranty, and dynamic pricing.
Drive growth while improving your performance using Microsoft Dynamics 365 with Alithya's proven expertise in mixed-mode manufacturing sectors, such as paper, rubber, plastics, and metals. Empower every area of your business to seize advantages beyond the next disruption by gaining clear insight and effective collaboration from one source of truth.
As a mixed-mode manufacturer, you face the challenges of both process and discrete production environments. Rely on our experience in both areas to help your organization achieve its business outcomes.
Enrich data that flows securely across all points of care ‒ giving healthcare providers the support they need to improve patient engagement and community health outcomes. Improve patient engagement, physician referrals, and health outcomes by flowing enriched business intelligence and CRM data securely through every point of care with integrated applications and analytics-driven dashboards on a single platform.
Alithya 365 Power Apps for Healthcare
Track patient and physician referrals, tie phone call info to marketing campaigns, and monitor the community health continuum.
- Alithya 365 Physician Referral Management
- Alithya 365 Patient Outreach
- Alithya 365 Marketing Analytics
- Alithya 365 Facilities Management
Boost productivity, reduce administrative tasks, optimize strategic outcomes, and streamline operations with automation that’s based on seamless integration of data-driven dashboards, tools, and agent, broker, and customer portals.
- Alithya 365 Power Apps for Insurance Brokers
Onboard and manage your insurance agents efficiently and reduce risks by staying complaint - AI-FI Trade Surveillance
Manage complex surveillance and compliance challenges with greater ease. With AI-FI Trade Surveillance, you can reduce false positives by up to 95% and spend more time on what’s important to your business and clients.
Exceed expectations with a true 360-degree view of your clients by enhancing Microsoft Dynamics 365 with Alithya’s professional services experts. Make the biggest difference to your clients and business with a framework that accelerates the ROI of your investment in Microsoft Dynamics 365. Achieve business outcomes such as increased bid win ratio, improved operational efficiency, more profitable projects, better account management and upselling, increased leads and stronger pipeline, and more.
Alithya 365 Property Management
Strengthen tenant relationships, create consistent leasing experiences, and deliver proactive maintenance services.
Elevate the performance of your entire business ‒ from the factory floor to customer service ‒ while addressing compliance, production, and sales challenges. Navigate erratic demand, resource and capacity constraints, strict regulations, and raw material volatility by empowering employees with accurate forecasts, real-time intelligence, best-practice templates, and modern ERP and CRM solutions.
Alithya solutions for all manufacturers:
- Alithya 365 Operations: Consolidate the information you need so you can gain key visibility on quality problems and drive important decisions in your organization to ensure compliance with requirements.
- Alithya 365 Power Apps for Manufacturing: Give your team higher-quality data to plan better, sell more, track assets and warranties, and process returns with our Microsoft Power Apps for manufacturing.
- Alithya 365 DiscreteXpress: Use a pre-configured infrastructure, toolset, and sample data to provide an easy place for discrete goods manufacturers to start their implementation—eliminating costly hours of analysis and training. The result: easily manage production orders, support lean manufacturing processes, forecast sales, configure products and more.
- Alithya 365 EAMXpress: Incorporate leading industry scenarios into a tested and proven asset management template for a quick shop-floor ERP implementation in equipment-focused industries. The result: streamline processes like corrective and preventative maintenance as well as spare parts management.
Alithya solutions by subvertical:
Microsoft Dynamics 365 and our industry expertise can empower you to conquer the challenges of producing and distributing high-quality food. Weather changing consumer preferences, tightening regulations, and rising demand for sustainability with a data-driven culture running on one source of truth.
Take on any challenge, including:
- Regulatory requirements
- Changing consumer demands
- FSA/USDA validation
- Recall and mock recalls
- Quality control
- Mergers and acquisitions
- Traceability
- Supply chain disruptions
Alithya 365 FoodXpress: Integrate more than 1,000 defined processes, data sets and food industry-related settings into Microsoft Dynamics 365 for F&O. The result: better manage batches and lots, co/by-product and catch weight processing, trade promotions, customer rebates and more.
Alithya 365 Dairy: Fulfill dairy-specific regulatory requirements, complex accounting and reporting rules, quality testing demands, traceability needs, and complex formulations/processing requirements.
Manage multiple variables across your product engineering and delivery processes by combining Microsoft Dynamics 365 with industry expertise that chemical manufacturers trust.
Solve challenges, including:
- Growing from mergers and acquisitions
- Changing regulations
- Minimizing raw materials waste
- Managing batch potency
- Accelerating research and development
- Developing sustainable products
Alithya 365 ChemXpress: Streamline chemical-specific procedures and leverage the benefits of your historical data for quick reporting across your organization. Results: easily manage batches and lots, potency, quality, co/by-product processing, customer rebates, trade promotions, compliance tracking, attribute-based pricing and more.
Alithya and Microsoft Dynamics 365 can help you move goods faster with less waste and better compliance. Maximize capacity, comply with regulatory demands, and drive continuous improvement by gaining greater visibility organization-wide with one source of truth.
Tackle these areas:
- Regulatory requirements
- FDA and USDA validation
- Quality controls
- Complete traceability
- Production and R&D management
- 21 CFR Part 11 electronic records
- Sarbanes-Oxley (SOX) compliance
- Good Automatic Manufacturing Practices issue 5 (GAMP 5)
- Security and compliance single sign-on
- Customer-specific requirements
- Expensive R&D process and high production costs
- Counterfeit products
- Supply chain disruptions
- Cyber security concerns
Alithya 365 PharmaXpress: Implement your ERP system properly to meet the needs of unique government regulations for pharmaceutical manufacturers and keep the thorough records needed to say compliant. Results: improved quality management, batch/lot management, compliance tracking, production dispensing and more.
Deliver high-quality products that ensure optimal patient outcomes by extending Microsoft Dynamics 365 with industry expertise that medical device manufacturers can trust. Get the full picture of your business in real time to anticipate and respond to supply chain risks and securely provide innovative life-saving medical devices.
Solve top challenges, including:
- Regulatory requirements and validation
- Complete traceability and quality controls
- Expensive R&D process and high production costs
- Counterfeit products
- Supply chain disruptions
- Cyber security concerns
- 21 CFR Part 11 electronic records
- Sarbanes-Oxley (SOX) compliance
- Good Automatic Manufacturing Practices issue 5 (GAMP 5)
Alithya 365 MedDeviceXpress: Combine lean manufacturing processes with strict regulatory compliance processes to succeed in the complex medical device manufacturing industry. The result: deploy an ERP system on a tighter timeline to meet these requirements and more.
Capture new sources of growth with operational agility and sustainability by blending Microsoft Dynamics 365 with expertise that building products manufacturers trust. Protect your revenue streams and profitability with data-driven smart manufacturing processes and business models supported by one source of truth.
Face your challenges, including:
- Construction market volatility
- Supply chain disruptions
- Tariff changes
- Production planning
- Inventory management
- Quality control
- Sustainability reporting
- Mergers and acquisitions
Keep up with volatile economic growth and surging demand by optimizing Microsoft Dynamics 365 with proven expertise for industrial goods manufacturers. Satisfy customer demand and bring smart manufacturing to life with Microsoft Dynamics 365 and warranties, configuration, Lean, advanced engineering change orders, product lifecycle management, enterprise asset management, field services, and more.
Microsoft Dynamics 365 can help you tackle common challenges like:
- Surging global competition
- Never-ending supply chain disruptions
- Emerging skill gaps
- Reshoring strategies
- Changing consumer demand
- Ongoing cybersecurity
- Needing to embrace new technology
Close more deals, track services, and manage warranties with an integrated Microsoft solutions that covers operations, field service, sales, customer service, project management, and marketing activities. Leverage data more effectively to strengthen relationships with customers and equipment manufacturing partners with relevant opportunities for ongoing growth.
Overcome top challenges, including:
- Labor shortages
- Equipment downtime
- Supply chain disruptions
- Inventory management and services
- Customer relationship management
- Surging demand for new and used equipment
- Lack of adoption of the latest technology
Alithya 365 Power App for Equipment Dealers: Leverage AI and data to analyze equipment sales and service history to target the right prospects and close more business.
Alithya 365 Advanced Field Service: Configure, price, quote and fulfill complex orders based on inventory and build-to-suit configured products, including parts, warranty, and dynamic pricing.
Drive growth while improving your performance using Microsoft Dynamics 365 with Alithya's proven expertise in mixed-mode manufacturing sectors, such as paper, rubber, plastics, and metals. Empower every area of your business to seize advantages beyond the next disruption by gaining clear insight and effective collaboration from one source of truth.
As a mixed-mode manufacturer, you face the challenges of both process and discrete production environments. Rely on our experience in both areas to help your organization achieve its business outcomes.
Enrich data that flows securely across all points of care ‒ giving healthcare providers the support they need to improve patient engagement and community health outcomes. Improve patient engagement, physician referrals, and health outcomes by flowing enriched business intelligence and CRM data securely through every point of care with integrated applications and analytics-driven dashboards on a single platform.
Alithya 365 Power Apps for Healthcare
Track patient and physician referrals, tie phone call info to marketing campaigns, and monitor the community health continuum.
- Alithya 365 Physician Referral Management
- Alithya 365 Patient Outreach
- Alithya 365 Marketing Analytics
- Alithya 365 Facilities Management
Boost productivity, reduce administrative tasks, optimize strategic outcomes, and streamline operations with automation that’s based on seamless integration of data-driven dashboards, tools, and agent, broker, and customer portals.
- Alithya 365 Power Apps for Insurance Brokers
Onboard and manage your insurance agents efficiently and reduce risks by staying complaint - AI-FI Trade Surveillance
Manage complex surveillance and compliance challenges with greater ease. With AI-FI Trade Surveillance, you can reduce false positives by up to 95% and spend more time on what’s important to your business and clients.
Exceed expectations with a true 360-degree view of your clients by enhancing Microsoft Dynamics 365 with Alithya’s professional services experts. Make the biggest difference to your clients and business with a framework that accelerates the ROI of your investment in Microsoft Dynamics 365. Achieve business outcomes such as increased bid win ratio, improved operational efficiency, more profitable projects, better account management and upselling, increased leads and stronger pipeline, and more.
Alithya 365 Property Management
Strengthen tenant relationships, create consistent leasing experiences, and deliver proactive maintenance services.
Modernize your business
From a single cloud to multi-cloud project, we have you covered. Get the most value from your Microsoft solutions with Alithya’s unparalleled implementation solutions, services, and expertise. Count on Alithya’s technology know-how and expertise for on-time, on-budget CRM and ERP implementations.
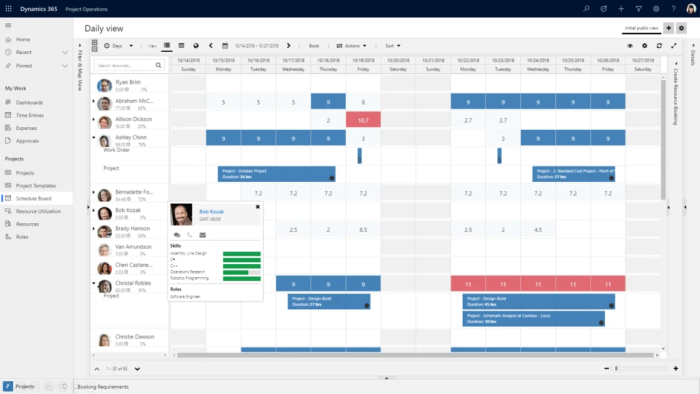
Microsoft Dynamics 365 Project Operations
From prospecting, to delivery, to payments, Project Operations helps you drive project success and profitability.
Plan, collaborate, manage and execute projects
Do you struggle with visibility, collaboration, and agility needed to drive success across your project-centric business? Or with successfully completing capital projects on time and on budget? Alithya is a premier partner for Microsoft Dynamics 365 Project Operations - a comprehensive solution that brings together sales, resourcing, project management, and finance teams within a single application.
It empowers business users to manage the entire project life cycle, from sales to invoicing and accounting. The software is designed for billable project-based organizations or organizations who are rolling out capital projects such as a new location or service. It provides tools for project contracts, execution, time tracking, and invoicing.
Finally, all your project needs in one place

Say goodbye to scattered business process management tools with high-technology solutions. Project Operations consolidates all your project-related requirements into a unified platform. This advanced technology simplifies project management and enhances collaboration and efficiency across your entire business operations team.
Dynamics 365 Project Operations is an evolution of Microsoft’s existing project management offerings, combining the power of:
- Microsoft Planner
- Project Online
- Project Service Automation (PSA)
- Dynamics 365 Finance
While these apps have specific strengths such as scheduling, time management, and accounting, Project Operations brings together all the relevant features under one umbrella. This allows you to plan, deliver, and scale projects without moving data from system to system.
Deliver more projects on time and on budget with Project Operations
Microsoft Dynamics 365 Project Operations helps you enhance customer satisfaction, streamline your workflow, and reduce costs. Take advantage of key features like advanced project planning, resource management, cost forecasting, and comprehensive analytics to enhance the overall efficiency and success of project-centric businesses.
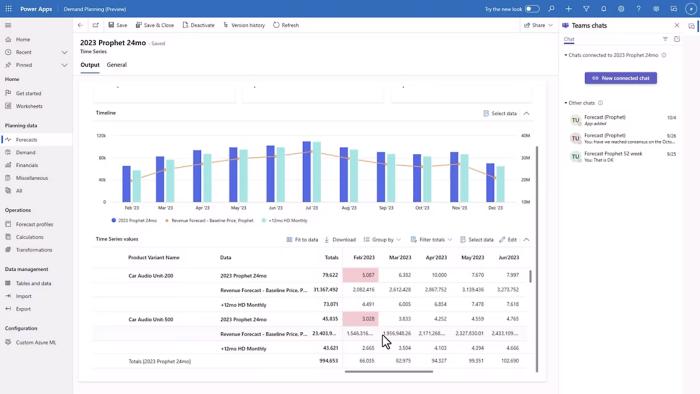
Forecast with precision
Accurate project forecasting is crucial for successful delivery. Project Operations provides advanced forecasting tools to help you make informed decisions, allocate resources efficiently, and ensure projects are delivered on time. Enable your team to prioritize contracts, identify risky opportunities and determine profitability.
Improve decision making
Informed decision-making relies on insightful analytics. With Project Operations, you gain access to detailed analytics, enabling you to assess project performance, identify trends, and find opportunities for improvement in your current systems. Visualize your project data through intuitive dashboards. Monitor progress, track key performance indicators, and stay informed at a glance to better inform decision-making.
Align your team
Effective communication is at the core of successful projects. Project Operations facilitates integration with Microsoft Teams for seamless communication in real-time, ensuring your team stays connected, informed, and aligned throughout the project lifecycle.
Increase productivity with AI
Microsoft Copilot for Project Operations helps automate actions using AI capabilities such as natural language processing. Replace manual tasks and redundant steps in current processes with business process automation to achieve operational efficiency.
Streamline projects with Alithya CoPlan
AlithyaCoPlan is an accelerator to Project Operations that helps finance and project teams to work together, by automating and streamlining the financials reconciliation process.
CFOs and PMOs often struggle to collaborate, which allows project budgets to slip. CFOs need up-to-date forecasts and financial data. PMOs spend days reconciling data and financials update, while managing complex projects. When all the data is finally gathered, it takes even more to manually reconcile all the entries to a single, digestible report.
Alithya’s innovative platform is designed to centralize and streamline project management and financial oversight for PMO and finance departments. It addresses critical challenges such as project prioritization, resource allocation, and budget management by providing up-to-date financial insights and real-time data.

With Alithya CoPlan, you can:
- Gain control and project agility
- Align projects with strategic goals
- Optimize resource use
- Adjust to budget variances quickly
- Improve forecasting accuracy
- Scale with ease to meet evolving business needs
- Connect data and people across platforms with robust integrations
- Make better decisions with real-time data
Accelerate modernization with Microsoft Power Platform
With our strategy and services, and Microsoft Power Platform features, your organization can automate and solve problems faster by connecting to Dynamics 365, Office 365, Azure, and hundreds of other apps.
Enhance apps and business processes with AI Builder, connect to a variety of data sources through Connectors, use Copilot and generative AI capabilities to boost productivity, along with powerful data service to quickly build enterprise-grade apps with Dataverse.
Microsoft Power Platform is a powerful suite of tools that can be used to address a wide range of business needs.
Automate processes
Power Automate can streamline repetitive tasks, such as employee onboarding, invoice processing, and customer service workflows.
Gain insights instantly
Power BI allows businesses to create interactive dashboards and reports, providing insights into sales performance, customer behavior, and operational efficiency.
Develop custom apps
Power Apps enables the creation of custom applications tailored to specific business needs, such as inventory management, project tracking, and field service operations.
Engage customers
Copilot Studio can be used to build AI-driven chatbots that handle customer inquiries, provide support, and improve overall customer satisfaction.
Connect all your systems
Power Platform can integrate with other Microsoft services as well as third-party applications to create seamless workflows and data exchanges.
Build low-code websites
Microsoft Power Pages empowers marketing and other non-technical employees with an intuitive platform that streamlines webpage building and management.
Automate everything with Power Automate
Power Automate helps you streamline your business processes and automate repetitive tasks. Its intuitive interface and many connectors allow you to create workflows with little to no knowledge of coding. You can drag and drop components and set up workflows to save time and improve efficiency. Power Automate can handle simple tasks like sending notifications as well as more complex processes across multiple apps and services. It's flexible and scalable, making it useful for various automation needs in a modern workplace.
Leverage Robotic Process Automation (RPA)

RPA simplifies building, deploying, and managing software robots that mimic human actions with digital systems. It connects humans with digital processes to end repetitive tasks - making digital transformation a reality. RPA uses AI and ML capabilities to handle high volume, repetitive tasks that previously required humans to perform.
RPA benefits include:
- Accelerated transformation
- No disruption to the underlying systems
- Minimal up-front investment
- Low-code build environment
- Scalable and enterprise-ready
- Higher accuracy
- Boosted productivity
- Cost savings
- Greater resilience
- More value from personnel
Build your own AI-driven copilots

Formerly Microsoft Power Virtual Agents, Copilot Studio is a graphical, low-code tool for both creating a custom copilot—including building automation with Power Automate—and extending a Microsoft 365 Copilot with your own enterprise data and scenarios.
A copilot is a powerful AI companion that can handle a range of interactions and tasks, from resolving issues requiring complex conversations to autonomously determining the best action to take based on its instructions and context. Copilots coordinate a collection of language models, along with instructions, context, knowledge sources, topics, actions, inputs, and triggers to accomplish your desired goals.
Copilots can engage with customers and employees in multiple languages across websites, mobile apps, Facebook, Microsoft Teams, or any channel supported by the Azure Bot Service. They can also improve productivity by performing tasks to assist users and organizations.
See the future with Power BI
Power BI is a collection of software services, apps, and connectors that work together to turn your unrelated sources of data into coherent, visually immersive, and interactive insights.
Your data might be an Excel spreadsheet, or a collection of cloud-based and on-premises hybrid data warehouses. Power BI lets you easily connect to your data sources, visualize and discover what's important, and share that with anyone or everyone you want.
Make it possible with Microsoft Power Apps
Power Apps is a suite of apps, services, and connectors, as well as a data platform, that provides a rapid development environment to build custom apps. These low-code or no-code apps connect to your data stored either in the underlying data platform (Microsoft Dataverse) or in many online and on-premises data sources (such as SharePoint, Microsoft 365, Dynamics 365, SQL Server, and so on).
Alithya 365 Powerhouse Playbook

Validate business value, gain internal buy-in, train citizen developers, and accelerate internal adoption of Power Apps. Build effective applications with confidence, thanks to Alithya’s governance and best practices guidance.
The Alithya 365 Powerhouse Playbook is our proven combination of advisory services, practical hands-on training, and Microsoft Power Apps that helps you:
- Educate and train your employees to leverage the Microsoft Power Platform with Power Apps
- Validate business value, gain internal buy-in and accelerate adoption of Power App projects
- Take advantage of coaching services to train users of all levels to create valuable low-code business Apps that respect best practices for compliance and security
- Gain visibility into who is building what and which data sources are being used
Industry-focused Alithya 365 Power Apps
Alithya's experts don't just know Microsoft, they also understand industry realities. This insight, combined with our client needs, has powered the development of our proprietary solutions.
Give your team the higher-quality data they need to plan better, sell more, track assets and warranties, and process returns.
Make account planning and business forecasting easier with Power Apps that bring together previously siloed data to provide a single view of customer behavior and market trends.
- Alithya 365 Smart Order Entry: Configure, price, quote and fulfill complex orders based on inventory and build-to-suit configured products, including parts, warranty, and dynamic pricing.
- Alithya 365 Advanced Field Service: Support customer serialized assets, track warranty claims/RMA, and add safety protocols to keep field technicians safe on the job.
- Alithya 365 Equipment Dealers: Close more deals and target the right prospects. This Power App leverages Dynamics 365 Sales and Field Service to enable tracking of equipment sales and service history. Leveraging Dynamics 365 Sales allows sales professionals to make decisions based on AI and data.
Track patient and physician referrals, tie phone call info to marketing campaigns, and monitor the community health continuum.
- Alithya 365 Physician Relationship Management (PRM): Improve your visibility into physician relationship management processes.
- Alithya 365 Patient Outreach: Track patient progress throughout the entire care continuum and see trends or patterns in patient health outcomes.
- Alithya 365 Marketing Analytics: Connect inbound patient phone calls to outbound marketing campaigns.
- Alithya 365 Facilities Management: Get a grip on warranty coverages, work order histories, claims processing and returns for hospital equipment.
- Alithya 365 Agency Management: Improve customer-facing interactions and arm brokers and agents with valuable data to manage and close business faster.
- Alithya 365 Power Apps for Insurance Brokers: Onboard and manage your insurance agents efficiently and reduce risks by staying complaint.
Start your digital transformation
Reach out to our Microsoft professionals to modernize your technology and processes for better business outcomes.